Coffee can’t be separated from the quality of the water used. Water, which takes up 90% of the space in espresso, dissolves the soluble components of the coffee beans. These include carbohydrates, lipids, acids, minerals, and proteins, as well as other nitrogenous compounds such as caffeine and trigonelline.
Coffee is a complex and rich beverage. Its components dissolve in different amounts and undergo different chemical reactions in each brew. While the most important factors affecting this dissolution and chemical reaction are the characteristics of the coffee bean, the effect of water is often underestimated. However, water composition drastically changes how much of these components in the ground coffee will be extracted, as well as affecting the taste of the water itself.
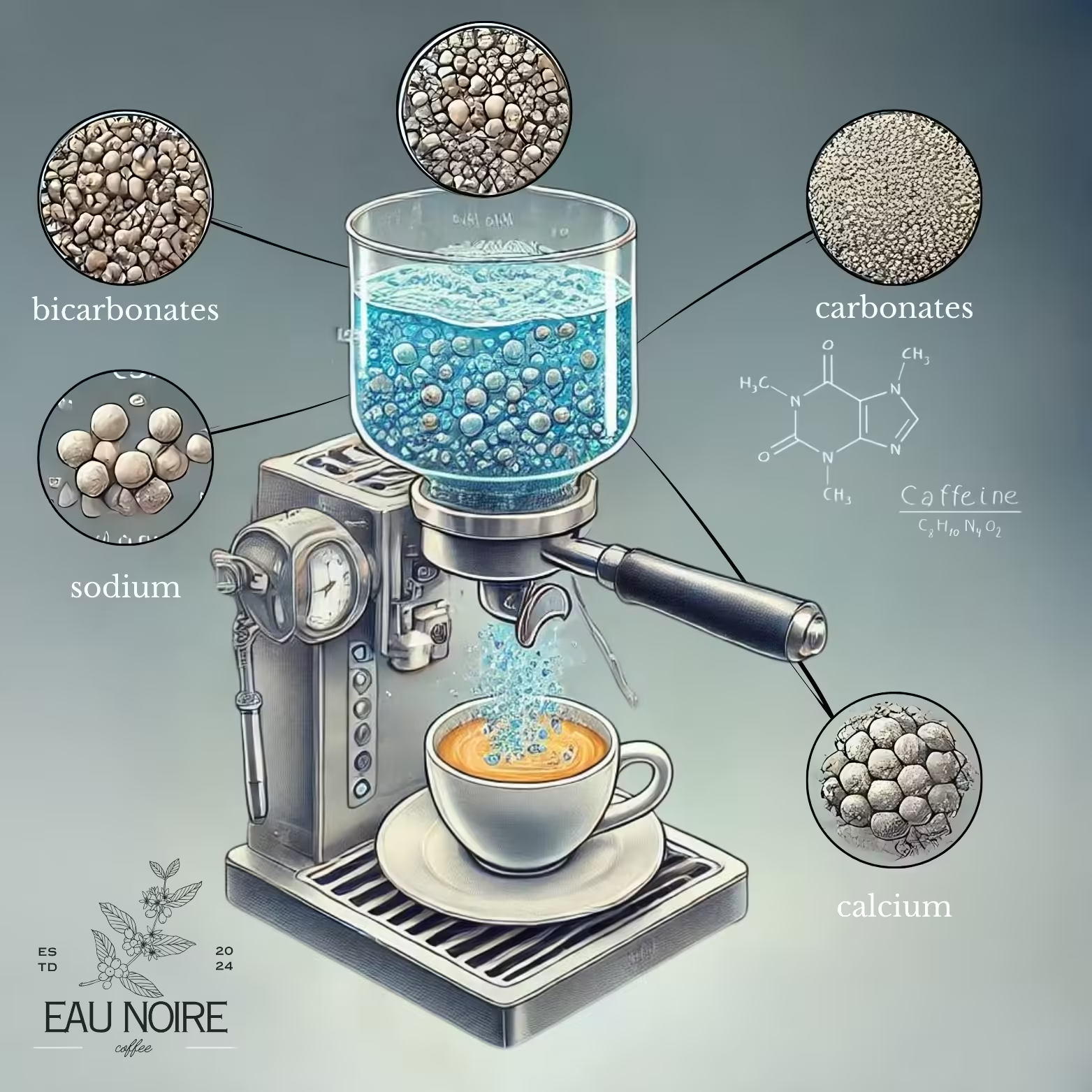
How does the water composition affect the coffee?
For instance, high levels of ions such as carbonate and bicarbonate can give coffee a bitter, flat taste. This neutralizes the coffee’s natural acidity, making it taste bland and weak. At the same time, these minerals can prolong the brewing time, leading to an unbalanced extraction of coffee components. As a result, the flavor profile of the coffee is negatively affected, resulting in a beverage that lacks the expected richness. On the other hand, when using water that lacks minerals and has a very low alkalinity, the sourness of the coffee can become overly dominant. This is especially the case with brews made with distilled water, and causes the natural acidic properties of the coffee to be overemphasized, which can lead to an unpleasant, overly sour taste profile.
The effect of water in espresso is much more complex than in other coffees.
To date, academic research into the relationship between water and coffee dates back to the 1950s. The overwhelming majority of these studies have examined immersion or pour over methods and have produced results that we now take for granted. But when it comes to espresso, which requires high pressure and temperature, the effects of water on coffee that have been discovered to date changes significantly.
Welcome to EAU NOIRE. I’m Oğuz Namal. In this article, we will explore the relationship between espresso and water in the light of the academic research “Water Quality for Espresso Coffee” published by L. Navarini and D. Rivetti in the Food Chemistry Journal in 2009.
This research provides an in-depth look at how the composition of water affects the preparation of espresso, and provides important findings on the water quality that must be considered to achieve the perfect cup of espresso. You can jump to the topics I will cover in this article below.
Why does this study matter?
This study significantly advances espresso coffee research by addressing gaps in previous studies, particularly the lack of quantitative data on how water composition and treatment affect espresso brewing. While the impact of water hardness and negative aromas on espresso quality is well known, the role of water in crema formation has been largely ignored. This study not only reviews existing knowledge, but also provides new experimental data on how different water compositions affect crema quality, making it important for optimizing the role of water in espresso preparation.
How does water content affect espresso extraction time?
In 1995, a study was conducted using tap water, softened water and demineralized water to investigate the effects of ions in water on espresso brewing. The time taken to brew a 40 ml (1/6 cup or 1 1/3 oz) espresso cup with each type of water was calculated. Compared to tap water, brewing time increased by 10% with demineralized water and 53% with softened water.
Composition of Tap Water, Demineralized Water and Softened Water
Why does the brewing time change?
The increase in bicarbonate ions in the water (especially in softened water) significantly increases the interaction with the acidic components in the coffee. This interaction results in a drop in the pH of the water and the bicarbonate is converted into a concentrated carbon dioxide (CO2). This gas causes the particles in the coffee bed to swell and prolongs the brewing time.
How does other minerals affect extraction time?
Rivetti and his team conducted experiments using various alkaline solutions to better understand the ions that affect espresso brewing time. The effects of lithium, sodium and potassium carbonate, sodium and potassium bicarbonate, and lithium, sodium, potassium and calcium hydroxide solutions were compared with pure water (Milli-Q). The results showed that carbonates and bicarbonates prolonged the brewing time by 55%, while the effect of hydroxide ions was even more pronounced, reaching up to 67%, especially when monovalent cations (sodium, potassium, lithium) were used. In contrast, the effect of divalent cations (e.g. calcium) was less (19%).
These findings led them to re-evaluate the role of carbonate and bicarbonate ions in extending brewing time. The researchers suggested that when natural waters are used, an increase in the pH level of the water changes the structure of the coffee bed, which may prolong the brewing time. In particular, the swelling of water-insoluble polysaccharides present in roasted coffee reduced the porosity of the coffee bed, making it difficult for water to pass through, resulting in a longer brewing time. The experimental data obtained were categorized into three main groups by analyzing the particle size distribution of coffee powder in contact with different types of water and solutions: pure water and natural raw water, softened water and sodium bicarbonate/carbonate solutions, and sodium hydroxide solutions.

Influence of Water on Espresso Crema
How is espresso crema formed?
One of the most important and distinctive characteristics of espresso is the layer of foam that forms on top of the coffee. This foam is known as “crema” and consists of carbon dioxide trapped in the cell structure of the coffee beans during roasting. According to recent studies, the carbon dioxide content of freshly ground coffee ranges from 4.0 to 8.6 mg/g, with an average of 5.7 mg/g. Let me explain the mechanism of espresso foam formation: During espresso brewing, the carbon dioxide in the coffee bed dissolves in the water under high pressure and temperature, leading to a supersaturated state in the beverage.
Experiment with 4 different waters
Content of the water used in the experiment
- If you use Milli-Q ultrapure water or water low in bicarbonate ions (such as A and B water), you will get a lower foam volume in your espresso coffee. These waters minimize the release of carbon dioxide, which creates a finer-textured, more persistent foam that is more acceptable to consumers.
- On the other hand, if you use waters rich in bicarbonate ions, such as D water, more foam is initially formed. However, this foam quickly settles, has low persistence and is characterized by large bubbles, leading to an undesirable texture. This high bicarbonate content changes the interaction of the water with the coffee, leading to the release of more carbon dioxide, which increases the volume of the foam, but negatively affects the quality of the foam.
Result: How to choose the best water for espresso?
*As an amazon associate i earn from qualifying purchases. The links above marked with * symbol are affiliated.

